Instructions for Electronic Submission of Form FDA 2541 Food Canning , The registration Form FDA 2541 requires identifying a facility contact person FCP for the establishment being registered The FCP can use the electronic AF LACF system. AF IMT Form 2541 NAF Journal Voucher TemplateRoller, A The AF IMT Form 2541 is used by authorized personnel in the United States Air Force who handle Non Appropriated Funds Q What information is required on the AF IMT Form 2541 A The AF IMT Form 2541 requires information such as the date of transaction account numbers descriptions of the transaction and the amount of funds involved
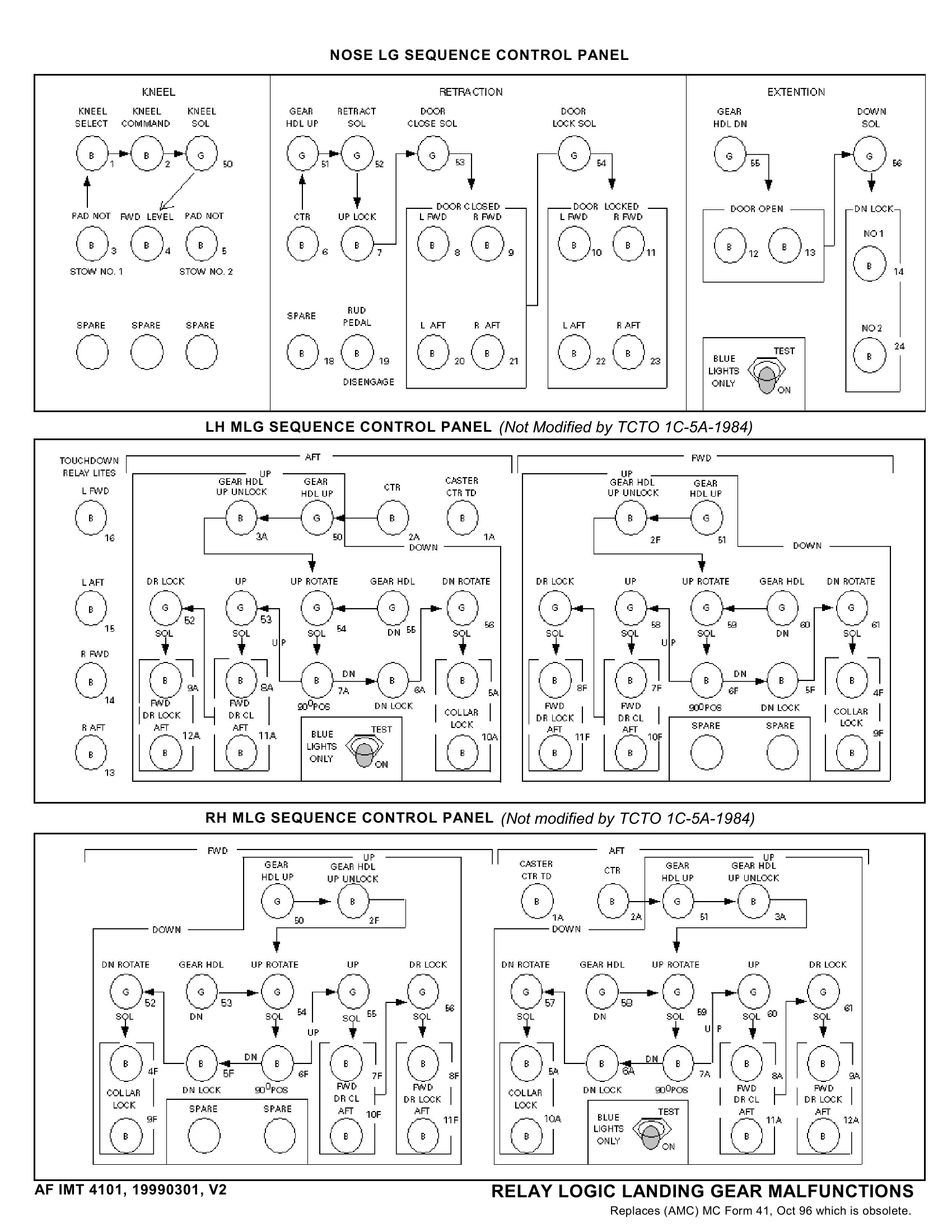
.Af Form 2541

Af Form 2541
Instructions for Electronic Submission of Form FDA 2541 Food Canning
The registration Form FDA 2541 requires identifying a facility contact person FCP for the establishment being registered The FCP can use the electronic AF LACF system.
span class result type PDF span Instructions for Electronic Submission of Form FDA 2541 Food Canning
You can submit Form FDA 2541 electronically or Submit Form FDA 2541 using a paper form tell us that you want to access the electronic AF LACF system when you send us your paper registration form and provide FDA with your FDA Account ID for the FIS electronic portal III How to Obtain an FDA Account a t the FIS Electronic Portal.

https://www.e-publishing.af.mil/Product-Index/
The official website for Air Force e Publishing Official websites use mil A mil website belongs to an official U S Department of Defense organization in the United States
https://www.fda.gov/media/86047/download
The registration Form FDA 2541 requires identifying a facility contact person FCP for the establishment being registered The FCP can use the electronic AF LACF system
span class result type PDF span Guidance for Industry U S Food and Drug Administration
Submitting Form FDA 2541 Food Canning Establishment Registration and Forms FDA 2541d FDA 2541e FDA V Changes to AF LACF Registration Information A Changing the Establishment Contact Person.
span class result type PDF span Please wait static e publishing af mil
Please wait If this message is not eventually replaced by the proper contents of the document your PDF viewer may not be able to display this type of document .
Submitting Food Canning Establishment Registration Form and Food
This guidance describes 1 Administrative procedures relating to the registration requirements of 21 CFR 108 25 c 1 for AF using Form FDA 2541 in both electronic and paper format 2 administrative procedures relating to the registration requirements of 108 35 c 1 for LACF using Form FDA 2541 in both electronic and paper format .
span class result type PDF span Instructions for Paper Submission of Form FDA 2541 Food Canning
Form FDA 2541 Food Canning Establishment Registration for an Acidified Low Acid Food Canning Commercial processors who manufacture process or pack acidified foods AF and or thermally processed low acid foods packaged in hermetically sealed containers historically referred to as low acid canned foods or LACF 1.
Disclaimer: All images featured on this web site are the property of their particular copyright owners. If you have any type of questions or worries regarding attribution or photo elimination, please do not hesitate to call us.